Shelf Life Determination of Pharmaceutical Products - Summary
Understanding the shelf life determination of pharmaceutical products is crucial for ensuring safety and effectiveness. All pharmaceutical drugs break down over time, creating by-products that may be harmful to the patients using them. The shelf life of pharmaceutical products refers to the time period during which the product keeps its identity and quality when stored under the conditions mentioned on the product label.
Importance of Shelf Life
Setting a clear time frame for the use of pharmaceutical products is very important. The ICH Q1E guideline provides valuable advice for estimating the shelf life of these products and substances. Determining shelf life involves careful evaluation of the stability data of a product.
Process of Determining Shelf Life
To estimate the shelf life of pharmaceutical products, at least three batches of data are usually required. For products kept at room temperature, assessments should start from the moment any significant changes are noticed in products stored under accelerated conditions. If no major changes occur in these accelerated conditions, then the shelf life will be based on data collected from long-term storage conditions.
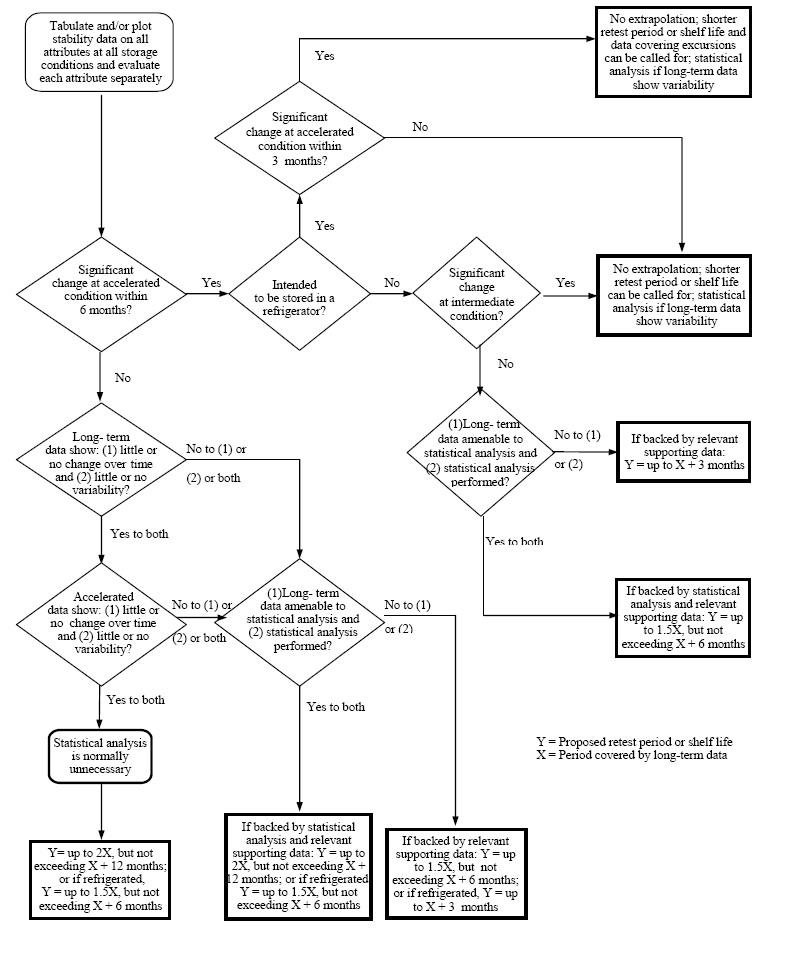 Shelf Life Chart
Shelf Life Chart
You can download the Shelf Life Determination of Pharmaceutical Products PDF using the link provided below.
