Periodic Table of Elements Chart - Summary
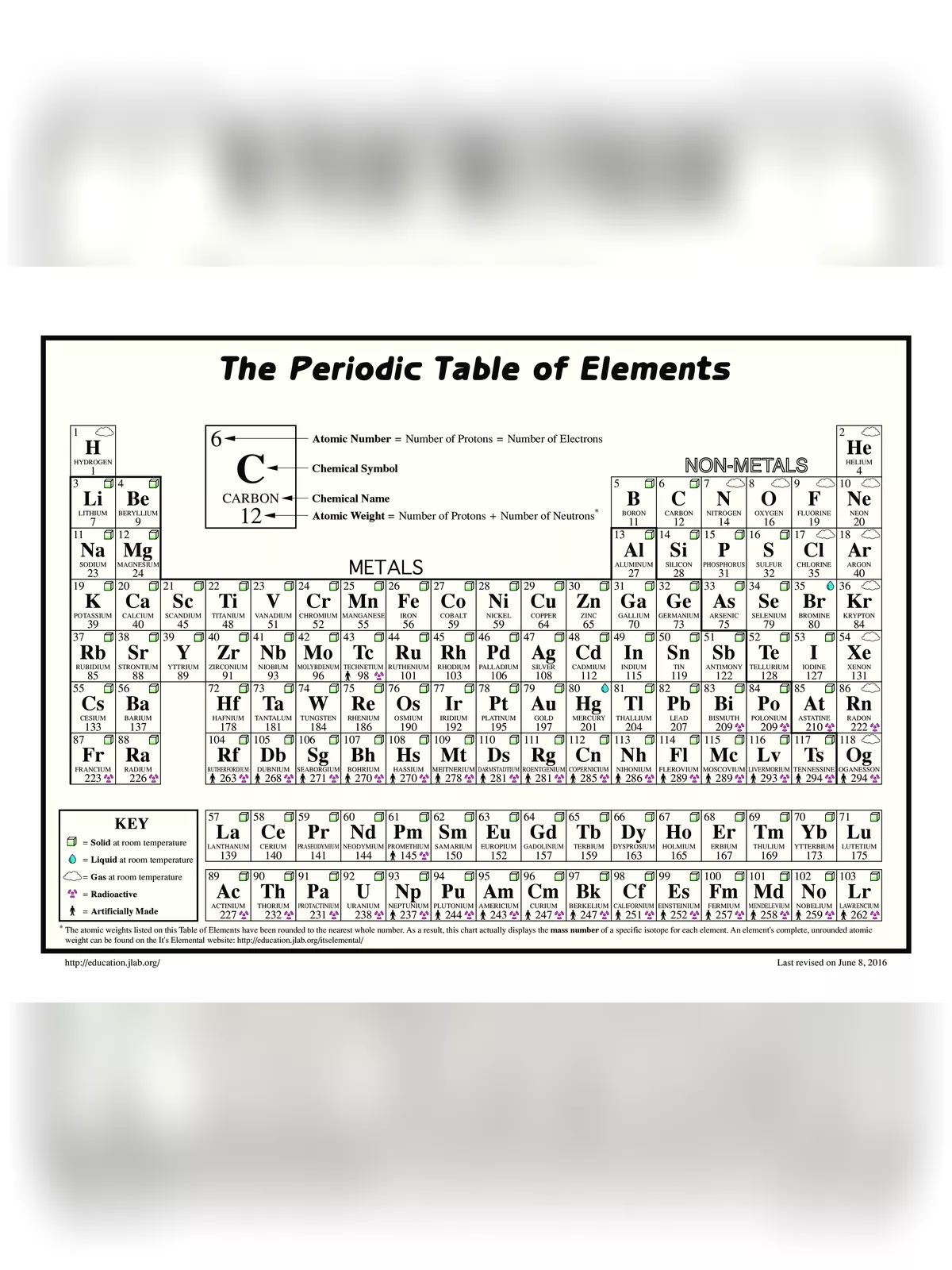
The Periodic Table of Elements is a very useful and easy-to-understand chart. It shows all the chemical elements arranged based on their atomic number. The smallest element is hydrogen, and it goes up to oganesson, which has the highest atomic number. The atomic number means the number of protons in the center of an element.
Understanding the Periodic Table PDF
The Periodic Table PDF helps you see how different elements are connected based on their properties. It has rows that go across, called periods. Think of it like floors in a building! The elements on the left are mostly metals, and the ones on the right are nonmetals.
What You Can Learn from the Periodic Table of Elements
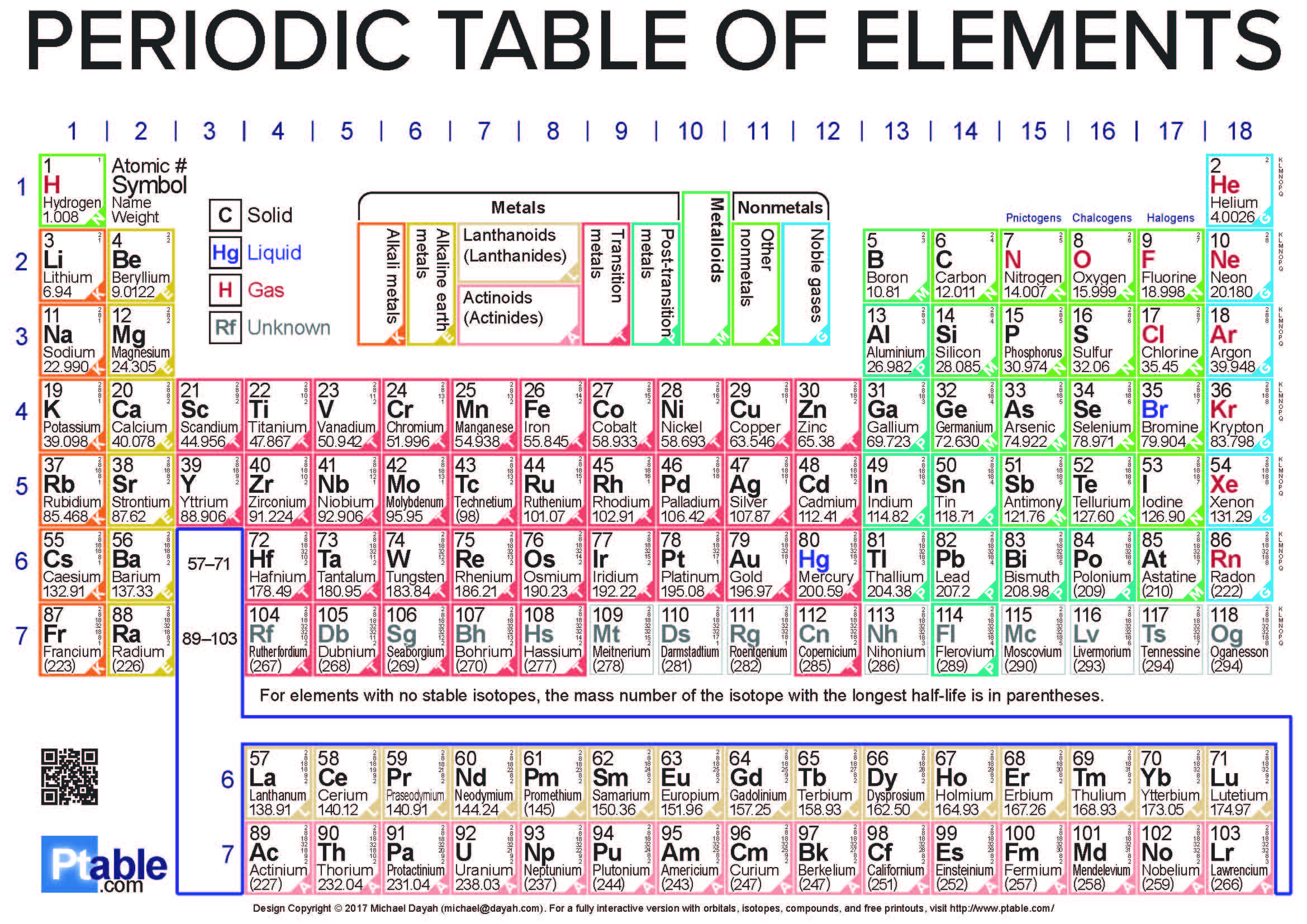
The columns, going from top to bottom, are called groups. Elements in the same group react in similar ways, like a family! There are six main groups, and each has its own special name and number. For example, the elements in group 17 are called halogens, and group 18 are the noble gases.
The table also has four sections called blocks – s, p, d, and f. This shows how the tiny electrons are arranged in the elements. Knowing about these blocks helps you understand why elements behave the way they do.
In 2025, the Periodic Table is complete up to element 118, Oganesson. Scientists are still searching for new elements, and if they find one, it will be added to the table!
Get Your Periodic Table Elements PDF Download for 2025
If you want to keep all this important information for studying anytime, you can download the updated Periodic Table of Elements PDF. This makes learning about elements very easy for students and anyone who loves science!